Overview
This guideline covers preventing bacterial infection in healthy babies of up to and including 28 days corrected gestational age, treating pregnant women whose unborn baby is at risk of infection, and caring for babies of up to and including 28 days corrected gestational age with a suspected or confirmed bacterial infection. It aims to reduce delays in recognising and treating infection and prevent unnecessary use of antibiotics. The guideline does not cover viral infections.
Last reviewed: 10 December 2025
We reviewed this guideline and will consider making recommendations or updating existing recommendations on:
- prelabour rupture of membranes at term, as a risk factor for early onset neonatal infection
- switching from intravenous to oral antibiotics for babies with suspected early onset neonatal infection.
See the guideline in development page for progress on the update.
This guideline updates and replaces NICE guideline CG149 (August 2012), which was called neonatal infection: (early onset): antibiotics for prevention and treatment.
Recommendations
This guideline includes new and updated recommendations on:
- preventing early-onset neonatal infection before birth
- risk factors for and clinical indicators of possible early-onset neonatal infection and late-onset neonatal infection
- investigations before starting antibiotics in babies who may have early-onset infection and late-onset infection
- antibiotics for late-onset neonatal infection
- duration of antibiotic treatment for late-onset neonatal infection
- antifungals to prevent fungal infection during antibiotic treatment for late-onset neonatal infection
- early- and late-onset meningitis in babies in neonatal units
It also includes recommendations on:
- antibiotics for suspected early-onset infection
- duration of antibiotic treatment for early-onset neonatal infection
- avoiding routine use of antibiotics in babies
- therapeutic drug monitoring for babies receiving gentamicin
- care setting
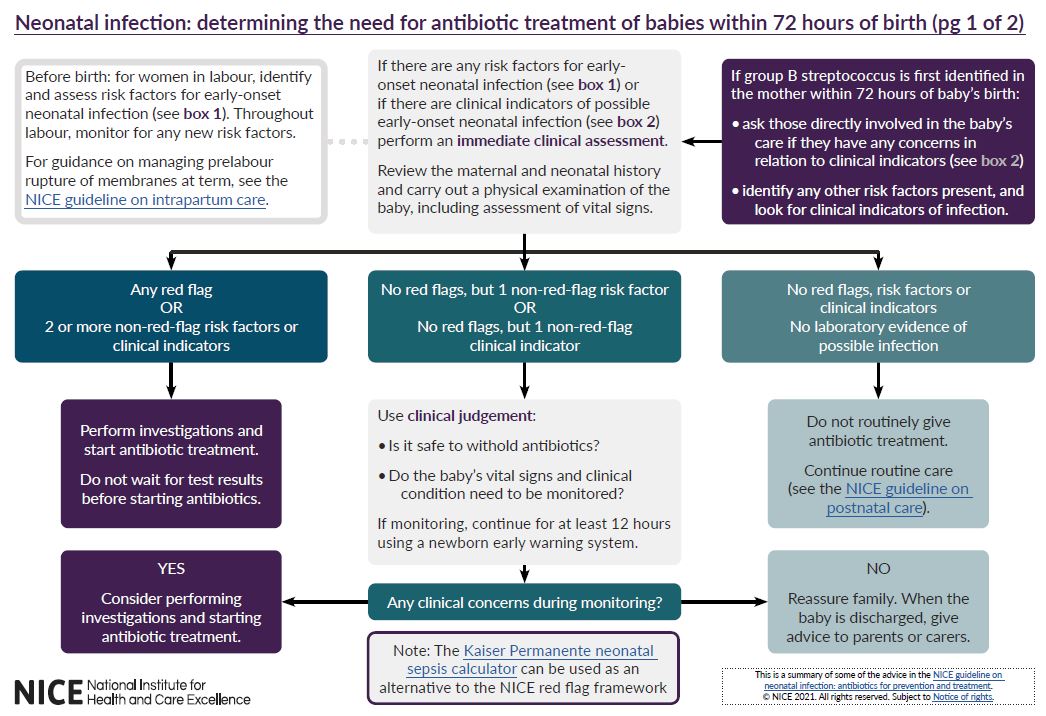
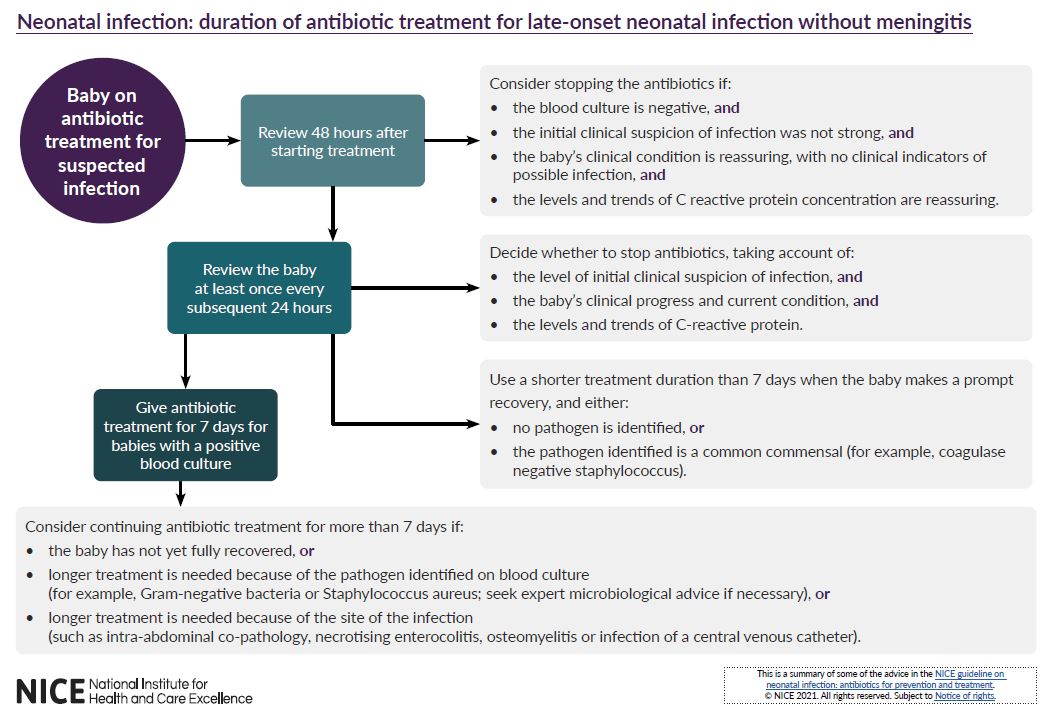
See visual summaries on the recommendations for determining the need for antibiotic treatment of babies within 72 hours of birth and duration of antibiotic treatment for late-onset neonatal infection without meningitis.
Who is it for?
- Healthcare professionals in primary and secondary care
- Commissioners and providers of neonatal and maternity services
- Parents and carers of babies who are at risk of or who have a neonatal infection
Guideline development process
How we develop NICE guidelines
This guideline was commissioned by NICE and developed in partnership with the Royal College of Obstetricians and Gynaecologists (RCOG)
Your responsibility
The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals and practitioners are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or the people using their service. It is not mandatory to apply the recommendations, and the guideline does not override the responsibility to make decisions appropriate to the circumstances of the individual, in consultation with them and their families and carers or guardian.
All problems (adverse events) related to a medicine or medical device used for treatment or in a procedure should be reported to the Medicines and Healthcare products Regulatory Agency using the Yellow Card Scheme.
Local commissioners and providers of healthcare have a responsibility to enable the guideline to be applied when individual professionals and people using services wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with complying with those duties.
Commissioners and providers have a responsibility to promote an environmentally sustainable health and care system and should assess and reduce the environmental impact of implementing NICE recommendations wherever possible.