Overview
This guideline covers diagnosing and managing chronic heart failure in people aged 18 and over. It aims to improve diagnosis and treatment to increase the length and quality of life for people with heart failure.
NICE has also produced a guideline on acute heart failure.
Last reviewed: 3 September 2025
We reviewed the evidence on treating and monitoring heart failure with reduced ejection fraction, mildly reduced ejection fraction and preserved ejection fraction. We amended the recommendations on heart failure with reduced ejection and added new recommendations on heart failure with mildly reduced and preserved ejection fraction. For more details, see the update information.
This guideline updates and replaces NICE guideline CG108 (August 2010).
Next review: This guideline will be reviewed if there is new evidence that is likely to change the recommendations.
How we prioritise updating our guidance
Decisions about updating our guidance are made by NICE’s prioritisation board. For more information on the principles and process, see NICE-wide topic prioritisation: the manual.
For information about individual topics, including any decisions affecting this guideline, see the summary table of prioritisation board decisions.
Recommendations
This guideline includes new and updated recommendations on:
- treating heart failure with reduced ejection fraction
- treating heart failure with mildly reduced or preserved ejection fraction
- starting and monitoring medication use
These supplement the existing recommendations on:
- multidisciplinary working
- diagnosing heart failure
- giving information for people with heart failure
- treating heart failure with chronic kidney disease
- cardiac rehabilitation
- palliative care
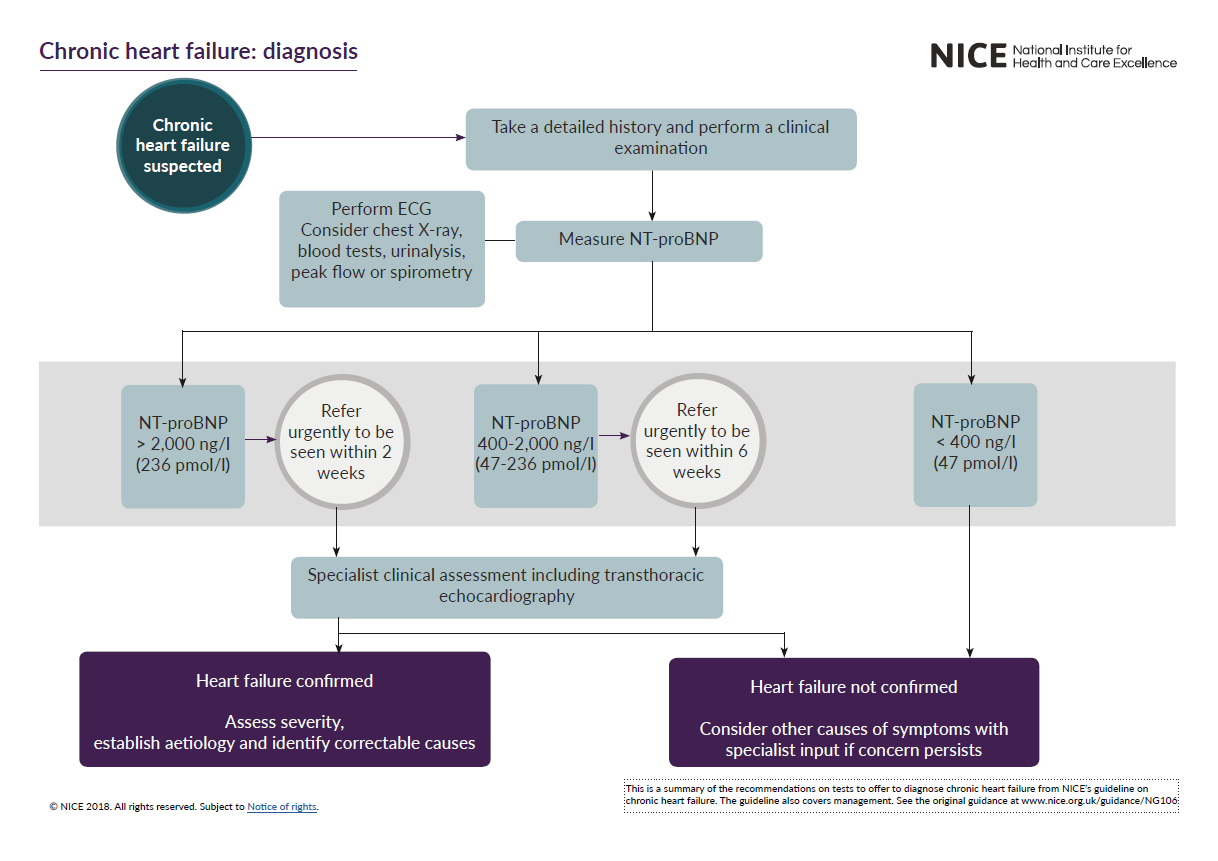
See the 1-page visual summary for diagnosing chronic heart failure and the 2-page visual summary on core treatments for chronic heart failure.
Who is it for?
- Healthcare professionals
- People with heart failure and their families and carers
Guideline development process
How we develop NICE guidelines
Your responsibility
The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals and practitioners are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or the people using their service. It is not mandatory to apply the recommendations, and the guideline does not override the responsibility to make decisions appropriate to the circumstances of the individual, in consultation with them and their families and carers or guardian.
All problems (adverse events) related to a medicine or medical device used for treatment or in a procedure should be reported to the Medicines and Healthcare products Regulatory Agency using the Yellow Card Scheme.
Local commissioners and providers of healthcare have a responsibility to enable the guideline to be applied when individual professionals and people using services wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with complying with those duties.
Commissioners and providers have a responsibility to promote an environmentally sustainable health and care system and should assess and reduce the environmental impact of implementing NICE recommendations wherever possible.