Overview
This guideline covers the early and longer-term (rehabilitation) management of acute coronary syndromes. These include ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI) and unstable angina. The guideline aims to improve survival and quality of life for people who have a heart attack or unstable angina.
This guideline does not cover management of spontaneous coronary artery dissection.
The recommendations in this guideline were developed before the COVID-19 pandemic. Acute coronary syndromes are a possible sign of acute myocardial injury in patients with COVID-19. See recommendations on acute myocardial injury in our rapid guideline on managing COVID-19.
Last reviewed: 19 December 2024
We added links to relevant technology appraisal guidance in the sections on dual antiplatelet therapy, coronary angiography with follow-on PCI, drug therapy for secondary prevention and people with reduced left ventricular ejection fraction. This is to provide easy access to relevant guidance at the right point in the guideline only and is not a change in practice.
This guideline updates and replaces:
- NICE guideline CG172 (published November 2013)
- NICE guideline CG167 (published July 2013)
- NICE technology appraisal guidance 230 (published July 2011)
- NICE guideline CG94 (published March 2010)
It incorporates unchanged NICE guideline CG130 (published October 2011).
It updates NICE technology appraisal guidance on drug-eluting stents for the treatment of coronary artery disease (TA152) and guidance on the use of coronary artery stents (TA71).
It also updates and replaces NICE medical technologies guidance MTG1 (published December 2010).
Next review: This guideline will be reviewed if there is new evidence that is likely to change the recommendations.
How we prioritise updating our guidance
Decisions about updating our guidance are made by NICE’s prioritisation board. For more information on the principles and process, see NICE-wide topic prioritisation: the manual.
For information about individual topics, including any decisions affecting this guideline, see the summary table of prioritisation board decisions.
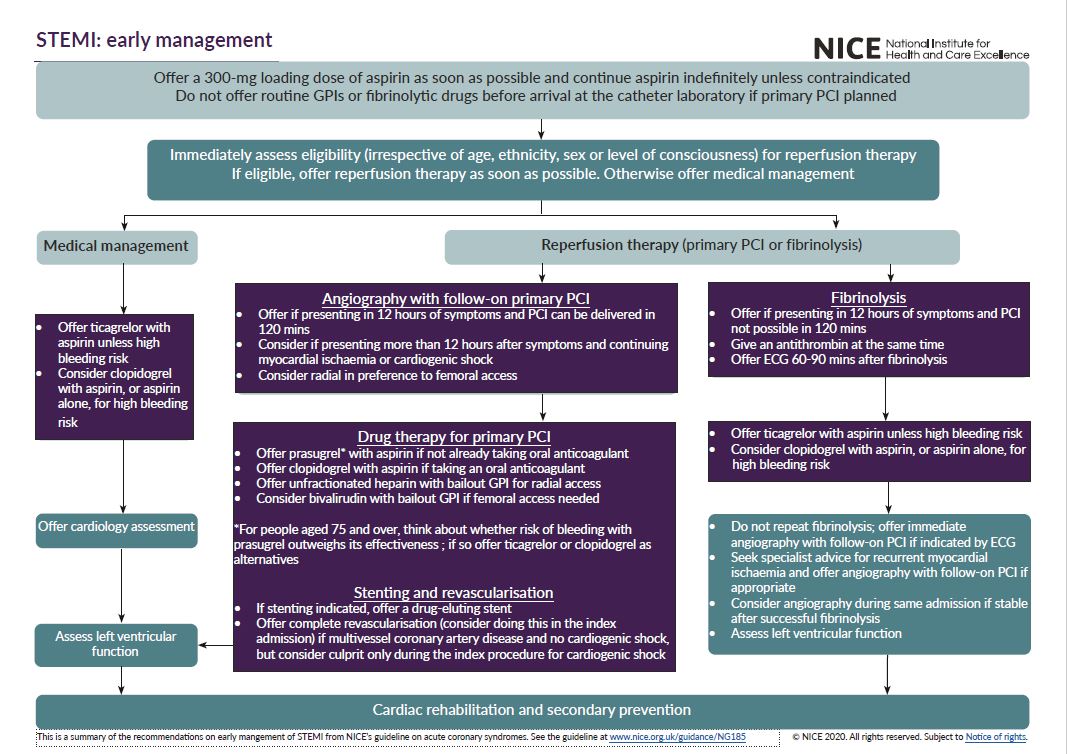
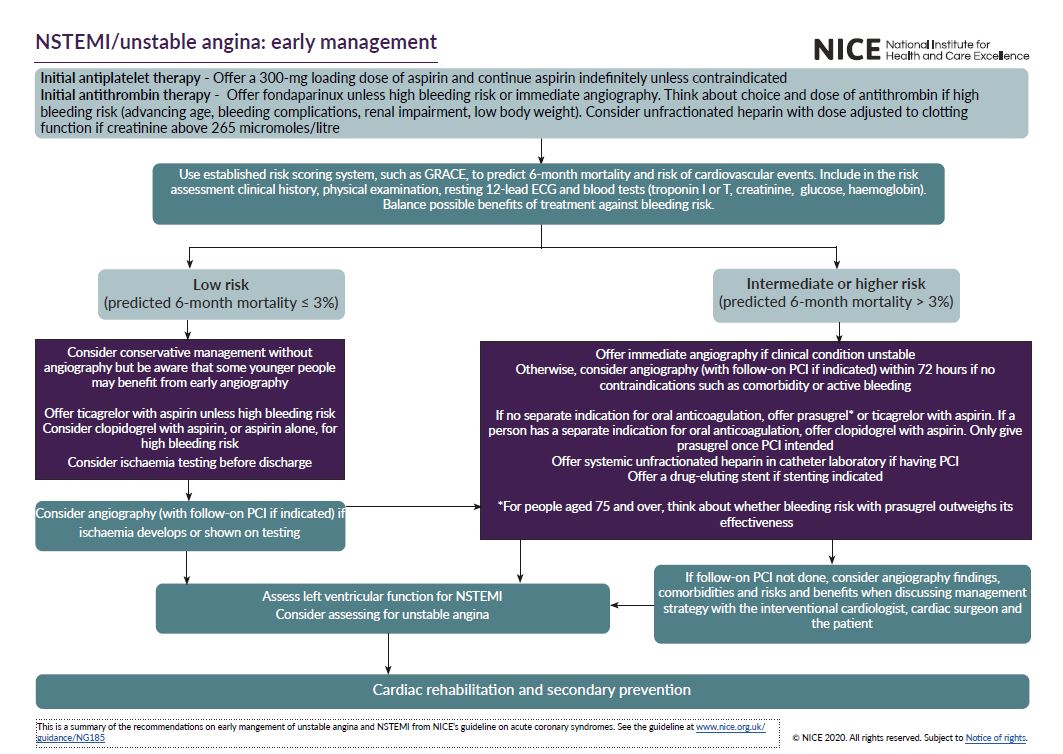
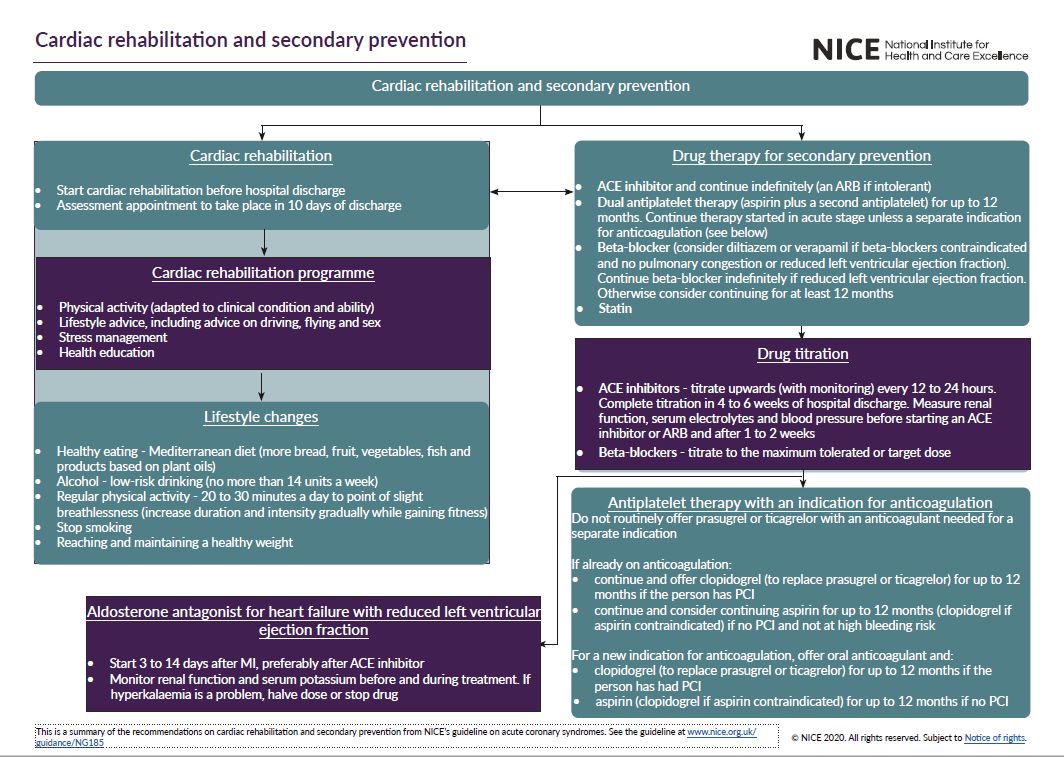
See the visual summaries on STEMI, unstable angina NSTEMI, and secondary prevention.
Recommendations
This guideline includes new and updated recommendations on:
- dual antiplatelet therapy and antithrombin therapy for primary percutaneous coronary intervention (PCI) for acute STEMI
- complete revascularisation versus culprit vessel-only PCI for STEMI
- drug-eluting stents for acute STEMI
- dual antiplatelet therapy for STEMI not treated with PCI
- initial drug therapy for unstable angina and NSTEMI
- coronary angiography with follow-on PCI for unstable angina and NSTEMI
- managing unstable angina and NSTEMI not treated with PCI
- combination antiplatelet and anticoagulant treatment as secondary prevention for people with a separate indication for anticoagulation
- duration of beta-blocker treatment for people with reduced left ventricular ejection fraction after myocardial infarction (MI)
These supplement the existing recommendations on:
- the early management of STEMI
- the early management of unstable angina and NSTEMI
- MI: secondary prevention and rehabilitation
Who is it for?
- Healthcare professionals
- Commissioners and providers
- Adults with acute coronary syndromes, their families and carers
Guideline development process
How we develop NICE guidelines
Your responsibility
The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals and practitioners are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or the people using their service. It is not mandatory to apply the recommendations, and the guideline does not override the responsibility to make decisions appropriate to the circumstances of the individual, in consultation with them and their families and carers or guardian.
All problems (adverse events) related to a medicine or medical device used for treatment or in a procedure should be reported to the Medicines and Healthcare products Regulatory Agency using the Yellow Card Scheme.
Local commissioners and providers of healthcare have a responsibility to enable the guideline to be applied when individual professionals and people using services wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with complying with those duties.
Commissioners and providers have a responsibility to promote an environmentally sustainable health and care system and should assess and reduce the environmental impact of implementing NICE recommendations wherever possible.